What is Inherited Thrombophilias?
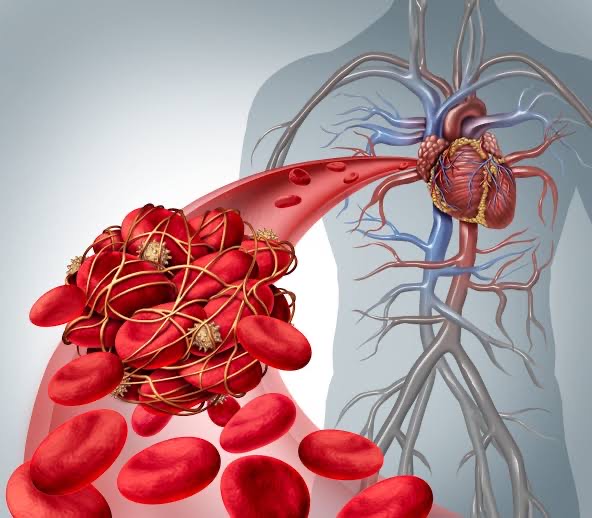
Inherited thrombophilias are a group of genetic conditions that increases the chance of developing abnormal clots (i.e., thrombosis) in the veins (blood vessels that carry blood from the body back to the heart) or arteries (blood vessels that carry oxygenated blood away from the heart to the body). A blood clot that obstructs or blocks a vein is called a venous thrombosis, and a blood clot that obstructs an artery is called an arterial thrombosis. Common sites for venous thrombosis include the veins deep inside the body (i.e., DVT: deep vein thrombosis which occurs when a blood clot develops in the large veins in the legs or arms), and in the lungs (PE: pulmonary embolism which occurs when a blood clot travels through the bloodstream and ends up in the lungs). DVTs and PEs are known as venous thromboembolism (VTE).
The Importance of F2 & F5 Gene
Inherited or hereditary thrombophilias are changes or mutations in the genes that are responsible for the body’s blood clotting system. The most common inherited thrombophilias are frequently caused by particular mutations in the F5 gene (called factor V Leiden) and the F2 gene. Mutations that cause deficiency of protein C, protein S, or antithrombin III also cause inherited thrombophilias. Mutations in these genes increase the chance of developing VTE.
The Clinical Expression of Thrombophilia by Contraceptives
The use of combined oral contraceptives (containing estrogen and progesterone) is common across the world. Unfortunately, their use has resulted in considerable adverse drug events that have caused much debate about their safety in the general population. One such adverse drug event that dates back to the early 1960s is the development of venous thromboembolism (VTE), which includes both deep vein thrombosis (DVT) and pulmonary embolism (PE). It has been estimated that the annual incidence of venous thromboembolism (VTE) increased 3-5 fold when women of childbearing age use estrogen-containing oral contraceptives. This risk is even greater in patients taking higher doses of estrogen.
The Science Behind the Test

A particular mutation in the F5 gene (i.e., factor V Leiden) allows the blood clotting process to remain active for a longer period of time, thus increasing the chance of developing abnormal blood clots. A mutation in the F2 gene (i.e. prothrombin G20210A) causes the gene to produce too much prothrombin. This can lead to the formation of abnormal blood clots. Mutations in these genes that cause deficiencies in these proteins can lead to the formation of abnormal blood clots. Inherited genetic factors predisposing the tendency to develop VTE are Factor V Leiden mutation in the F5 gene and mutation 20210G>A in the F2 (prothrombin) gene. F5 Leiden and prothrombin thrombophilia are inherited in an autosomal dominant manner, heterozygosity for the above-mentioned variants results in an increased risk for thrombosis; homozygosity for the variants confers a higher risk for thrombosis than heterozygosity.
Thrombophilia by Hormone Replacement Therapy
Hormone replacement therapy is not for everyone, but some people find the treatment provides relief from the symptoms. Hormone Replacement Therapy (HRT) is used for the management of menopausal symptoms such as hot flashes, dry skin, and vulvovaginal atrophy. A well-documented risk of using HRT includes an increased risk of deep vein thrombosis (DVT), which may even lead to blood clots in the lungs (Pulmonary Emboli, PE).
Inherited genetic factors predisposing tendency to develop VTE are Factor V Leiden mutation in the F5 &‘ F2 (prothrombin) gene. F5 Leiden and prothrombin thrombophilia are inherited in an autosomal dominant manner, heterozygosity for the above-mentioned variants results in an increased risk for thrombosis.
What We Test?
How it Works?
The process is as simple as ordering a kit from our lab. When it arrives after 3 business days, you will collect a saliva sample with a quick cheek swab provided in the kit. Then, just send it back in the prepaid mailer, and we will send it to our Lab in the USA. Your results will be ready in 2-4 weeks.
The average time is (3 weeks).
